Jun 4, 2024
Britain Poised to Offer Eli Lilly’s Weight-Loss Drug to Patients
, Bloomberg News
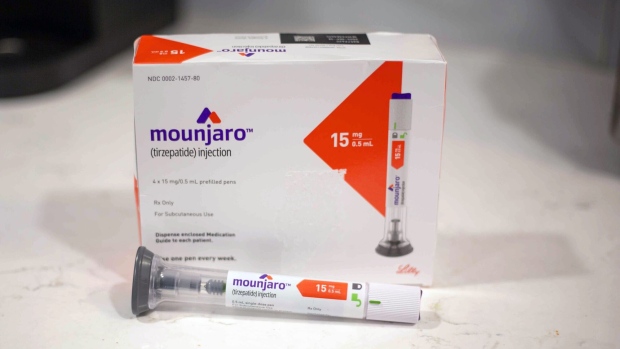
(Bloomberg) -- Eli Lilly & Co.’s Mounjaro is being recommended for weight loss for some UK patients with obesity and could potentially be easier to access on the National Health Service than Novo Nordisk A/S’s hit treatment Wegovy.
Patients with a body mass index of at least 35 and at least one weight-related co-morbidity should take Mounjaro, according to draft guidance issued by the UK’s drug cost regulator. People with a lower BMI threshold from several ethnic backgrounds could also take tirzepatide — known as Mounjaro in the UK and Zepbound in the US — it said.
If taken alongside diet and exercise support, Mounjaro might be more effective than Wegovy, the National Institute for Health and Care Excellence said.
Unlike the blockbuster Wegovy, patients on Mounjaro won’t need to be part of a specialist weight-management service to receive the drug, the regulator said. There is also unlikely to be a cap on how long a patient can take Mounjaro whereas Wegovy use on the NHS is capped at two years, it added.
Novo and Lilly are battling for the lead in a global obesity-drug market that Goldman Sachs has estimated may reach $130 billion by 2030. Though Lilly was second to the market, Goldman analysts suggested that the US company may have a slight advantage over its Danish rival.
The recommendations are still in draft form and could change following a consultation. If the guidance is confirmed, it potentially opens the door to cheaper access to weight-loss drugs for many patients.
Allowing patients to get the drug directly through their doctor is “likely to widen access to treatment,” said Nerys Astbury, associate professor of diet and obesity at the University of Oxford, in comments shared by the Science Media Centre.
In coming to its conclusions, the regulator heard evidence that other medicines such as semaglutide, or Wegovy, are only available to a relatively small group of people because of the requirement for attendance at specialist weight-management clinics, which are only accessible for a maximum of two years.
During the deliberations, a clinical adviser from NHS England as well as a patient expert told the regulator that the setting for Mounjaro shouldn’t be limited.
The regulator said patients on Mounjaro should consider stopping the drug if less than 5% of their initial weight has been lost after 6 months.
Mounjaro received backing from the cost regulator last year for treating patients with type 2 diabetes, paving the way for broader use of the drug for weight loss.
Both Mounjaro and Wegovy are already available privately in the UK for patients that meet certain criteria.
“This class of injectable drug is currently expensive, providing particular challenges to a taxpayer-funded health system like the NHS,” said Stephen O’Rahilly, professor of clinical biochemisty and medicine at the University of Cambridge.
--With assistance from Naomi Kresge.
(Updates with further detail and comments from paragraph 1.)
©2024 Bloomberg L.P.