Sep 19, 2022
Boston Scientific Workers Allegedly Called Out Misuse of Its Device and Were Ignored
, Bloomberg News
(Bloomberg) -- Boston Scientific Corp. ignored complaints from its employees that a national medical chain was performing unnecessary procedures with its devices, according to allegations contained within a federal lawsuit against the vascular clinics.
Boston Scientific isn’t named as a defendant in the lawsuit, a whistle-blower case against Modern Vascular that was filed in January 2020 and kept under seal until the US Department of Justice decided to intervene late last week. The suit in US District Court in Arizona alleges that the clinics performed unnecessary procedures and were engaged in an illegal kickback scheme to acquire patients, in violation of several federal health-care fraud laws.
Modern Vascular, which has clinics in 10 states according to its website, employed doctors who treated patients with blocked arteries in the legs and feet. In one type of procedure, they shaved plaque off artery walls using atherectomy devices such as those sold by Boston Scientific. In their lawsuit against Modern Vascular, doctors Jay Radhakrishnan and William Julien say that Boston Scientific representatives wrote internal memos about unnecessary procedures but supervisors and managers “turned a blind eye,” not wanting to hurt their sales of atherectomy devices.
A Boston Scientific spokesperson said the company “strongly” disagrees with the allegations.
“We conducted an investigation into these concerns and believe that no inappropriate conduct by Boston Scientific had taken place,” the Boston Scientific spokesperson said in a statement. Another spokesperson added that the company had previously been in touch with the DOJ about the matter and that “we have complied with all of their requests and will continue to do so.”
The case was consolidated with two other whistle-blower suits against Modern Vascular on Sept. 6. Whistle-blower complaints are generally kept sealed while the federal government decides whether or not to intervene in a case. The DOJ filed a motion on Sept. 15 notifying the court it decided to intervene, which led to this case being unsealed the next day.
Modern Vascular, in a statement, said the complaints were brought by competitors “who don’t know how our business operates” and that they have no merit. “We intend to vigorously defend against the claims,” the company said.
The company said its mission is to eliminate unnecessary amputations using education, behavior modification and medical management in addition to outpatient procedures. It said it continues to cooperate with the DOJ and the federal court. “Compliance is a top priority for us,” the company said.
In 2021, Boston Scientific reported $1.82 billion in sales of peripheral-interventions products, a category that includes these atherectomy devices, according to data compiled by Bloomberg. It is the only Boston Scientific business unit to have seen sales growth each year since 2018.
Atherectomy in leg arteries has grown in popularity. It is a financially lucrative procedure even though researchers argue there is limited evidence showing it is more effective than cheaper alternatives such as stents and angioplasty as an initial intervention for certain arterial diseases.
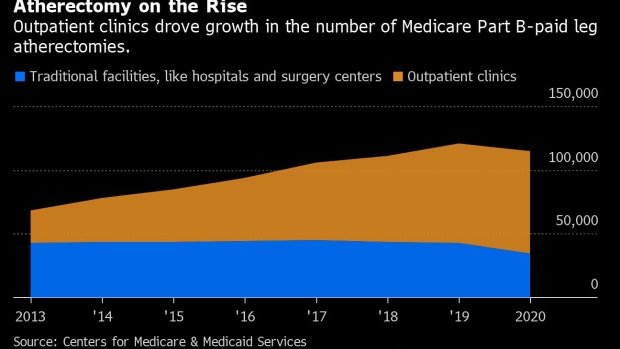
Since 2013, the number of leg atherectomy procedures paid for by Medicare Part B has increased steadily, with a slight dip in 2020, the first year of the Covid-19 pandemic, data from the Centers for Medicare & Medicaid Services show. All of that growth has occurred in outpatient clinics, where doctors can get paid more money for the procedure than in hospitals or surgery centers. In 2020, the most recent year for which data were available, Medicare paid for at least 115,006 leg atherectomy procedures, 69.7% of which were performed in outpatient clinics.
The case is Radhakrishnan v. Gampel, 20-cv-00176, US District Court, District of Arizona.
©2022 Bloomberg L.P.